KUVAN Established Safety Profile
- The efficacy and safety of KUVAN® (sapropterin dihydrochloride) Tablets for Oral Use were evaluated in 6 clinical studies in patients with PKU1
- The safety of KUVAN has been tested in over 600 people with PKU ranging in age from 1 month to
50 years
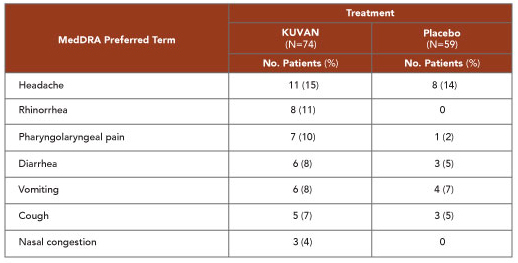
- The most common adverse reactions (incidence ≥4%) were headache, rhinorrhea, pharyngolaryngeal pain, diarrhea, vomiting, cough, and nasal congestion1
- The overall incidence of adverse reactions in patients receiving KUVAN was similar to that reported with patients receiving placebo1
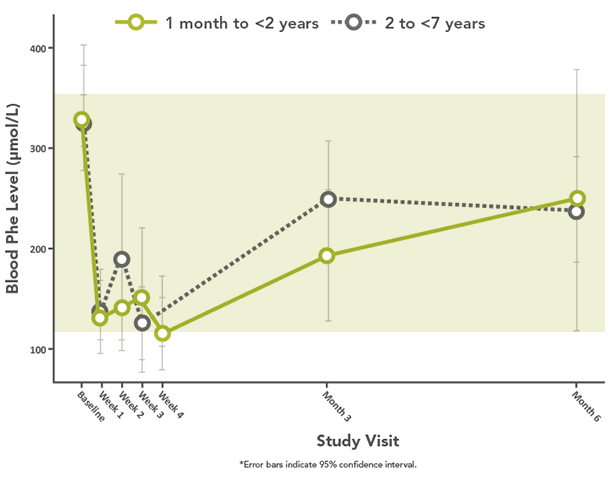
- Except for an increased incidence of low blood Phe levels in patients under 7 years of age
- In clinical studies, the safety profile of KUVAN has been established for pediatric patients as young as 1 month of age1
Summary of Adverse Reactions Occurring in ≥4% of Patients in Placebo-Controlled Clinical Studies With KUVAN® (sapropterin dihydrochloride)1
- Hypersensitivity reactions, including anaphylaxis, have occurred
- Monitor patients for signs of gastritis
- Children younger than 7 years of age treated with KUVAN doses of 20 mg/kg per day are at increased risk for low levels of blood Phe, compared with patients 7 years and older
- Active management of dietary Phe intake during KUVAN treatment is required
- Monitor patients for signs of hyperactivity
KUVAN Pharmacodynamics and Pharmacokinetics
- A clear dose-response relationship has been demonstrated for KUVAN® Tablets or Powder for Oral Solution
- In a forced dose-titration study, patients received KUVAN doses of 5, 10, and 20 mg/kg per day
(for 2 weeks at each dose)
- At the end of each 2-week period, blood Phe levels decreased as the dose of KUVAN increased
- For patients aged 1 month to 49 years, body weight is the only covariant affecting plasma clearance or distribution volume of KUVAN
Reference
- KUVAN [package insert]. Novato, CA: BioMarin Pharmaceutical Inc; 2021.
INDICATION
KUVAN® (sapropterin dihydrochloride) Tablets for Oral Use and Powder for Oral Solution are indicated to reduce blood phenylalanine (Phe) levels in adult and pediatric patients one month of age or older with hyperphenylalaninemia (HPA) due to tetrahydrobiopterin- (BH4-) responsive Phenylketonuria (PKU). KUVAN is to be used in conjunction with a Phe-restricted diet.
IMPORTANT SAFETY INFORMATION
Treatment with KUVAN should be directed by physicians knowledgeable in the management of PKU. Prolonged exposure to elevated blood Phe levels can result in severe neurologic damage in PKU patients.
The use of KUVAN does not eliminate the need for careful monitoring of blood Phe levels and ongoing dietary management to ensure adequate Phe control and nutritional balance. Response to KUVAN can only be determined by a therapeutic trial. Patients should be advised to notify their physicians in cases of overdose.
Warnings and Precautions
- Hypersensitivity Reactions Including Anaphylaxis: KUVAN is not recommended in patients with a history of anaphylaxis to KUVAN. Hypersensitivity reactions, including anaphylaxis and rash, have occurred. Signs of anaphylaxis include wheezing, dyspnea, coughing, hypotension, flushing, nausea, and rash. Discontinue KUVAN treatment in patients who experience anaphylaxis, and initiate appropriate medical treatment. Continue dietary Phe restrictions in patients who experience anaphylaxis.
- Upper Gastrointestinal Mucosal Inflammation: Gastrointestinal (GI) adverse reactions suggestive of upper GI mucosal inflammation have been reported with KUVAN. Serious adverse reactions included esophagitis and gastritis. If left untreated, these could lead to severe sequelae including esophageal stricture, esophageal ulcer, gastric ulcer, and bleeding, and such complications have been reported in patients receiving KUVAN. Monitor patients for signs and symptoms of upper GI mucosal inflammation.
- Hypophenylalaninemia: Some patients receiving KUVAN can experience significant drops in blood Phe levels, and children younger than 7 years old treated with KUVAN doses of 20 mg/kg per day are at an increased risk for low levels of blood Phe compared with children 7 years and older.
- Monitoring Blood Phe Levels During Treatment: Prolonged elevations of blood Phe levels in patients with PKU can result in severe neurologic damage, including severe intellectual disability, developmental delay, microcephaly, delayed speech, seizures, and behavioral abnormalities. Conversely, prolonged levels of blood Phe that are too low have been associated with catabolism and endogenous protein breakdown, which has been associated with adverse developmental outcomes. Active management of dietary Phe intake and frequent blood Phe monitoring while taking KUVAN is required to ensure adequate Phe control and nutritional balance, especially in the pediatric population.
- Lack of Biochemical Response to KUVAN: Not all patients with PKU respond to treatment with KUVAN. Biochemical response to KUVAN treatment cannot generally be pre-determined by laboratory testing (e.g., molecular testing), and should be determined through a therapeutic trial (evaluation) of KUVAN response.
- Interactions with Levodopa: In a post-marketing safety surveillance program for a non-PKU indication using another formulation of the same active ingredient (sapropterin), there have been reports of an interaction with levodopa causing seizures, exacerbation of seizures, over-stimulation, and irritability. Monitor patients who are receiving levodopa for a change in neurologic status during treatment with KUVAN.
- Hyperactivity: There have been post-marketing reports of hyperactivity with administration of KUVAN. Monitor patients for hyperactivity.
Adverse Reactions
- Most common: The most common adverse reactions (incidence ≥4%) were headache, rhinorrhea, pharyngolaryngeal pain, diarrhea, vomiting, cough, and nasal congestion.
- Additional adverse reactions reported in connection with worldwide marketing: hypersensitivity reactions including anaphylaxis and rash, esophagitis, gastritis, oropharyngeal pain, pharyngitis, esophageal pain, abdominal pain, dyspepsia, nausea, vomiting, and hyperactivity.
Additional Drug Interactions
- Frequently monitor blood Phe levels when co-administering KUVAN with medications known to inhibit folate metabolism, such as methotrexate, valproic acid, phenobarbital, trimethoprim.
- Monitor patients for hypotension when co-administering KUVAN with medications known to affect nitric oxide–mediated vasorelaxation such as PDE-5 inhibitors including sildenafil, vardenafil, or tadalafil.
To report SUSPECTED ADVERSE REACTIONS, contact BioMarin Pharmaceutical Inc. at 1-866-906-6100, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please read the full Prescribing Information by clicking here.